Science as
a tool.
People as
inspiration.
Innovation leaders in Latin America with all the attributes a CRO must master.
We work in with our clients to enable and regulate clinical research

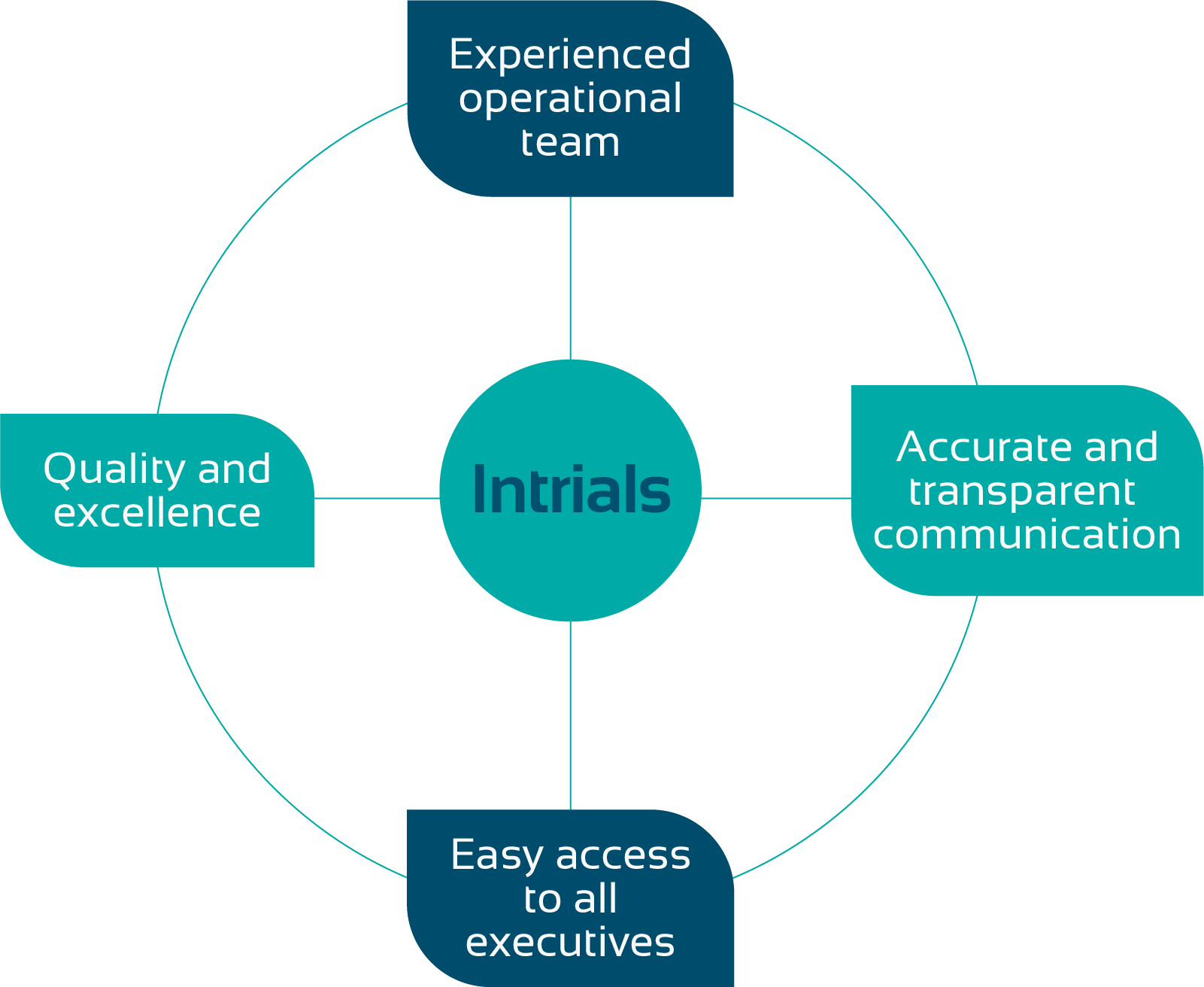
Where scientific experience meets professional excellence



25+ years in Latin America
550+ studies developed
Services
Read more
Full regulatory support to optimize approval times
Management
Read more
Customized projects in all phases: country-wide or internationally
Monitoring of
Clinical Studies
Read more
Local CRAs trained and supervised by experienced Project Managers
Quality control in conducting the clinical protocol, ensuring robust data and respect to well-being and rights of the participants
Assurance
Read more
Ensuring compliance across the project lifecycle in multiple countries
Ensuring quality in all processes

Regulatory
Services
Read more
Deep understanding of the country-specific process
Full regulatory support to optimize approval times

Management
Read more
Strong regional experience
Customized projects in all phases: country-wide or internationally

Monitoring of
Clinical Studies
Read more
Alignment with ICH-GCP and regional specificities
Local CRAs trained and supervised by experienced Project Managers
Quality control in conducting the clinical protocol, ensuring robust data and respect to well-being and rights of the participants

Quality
Assurance
Read more
Promoting excellence at all levels
Ensuring compliance across the project lifecycle in multiple countries
Ensuring quality in all processes
Why Latin America?
We are focused to offer your project the necessary expertise
in applying international quality standard to regional specificities
Multicultural
Field with more than 560 million people,
constantly growing and study options
in the presence of native patients
Communities and Minorities
Screening and enrollment of patients in clinical trials will continue to evolve to reach a greater number of patients considered minorities
Multicultural
Field with more than 560 million people,
constantly growing and study options
in the presence of native patients
Communities and Minorities
Screening and enrollment of patients in clinical trials will continue to evolve to reach a greater number of patients considered minorities
USA & UK
Equivalent quality standards
Continuing Growth
Above the global pharmaceutical market
Medication development phase (drugs)
| Phase l |
| Phase ll |
| Phase lll |
| Phase lV |
| Study Extension |
| Observational |
| Post-Study |
| Natural History Studies |
| Bioquivalence (BQV / BEq) |
| Regulatory Affairs |
| Patient Recruitment Assistance |
| Feasibility Assessment |
| Protocol Creation and Study Strategies |
| Clinical Trial Management |
| Safety Management (SAE) |
| Clinical Study Report and Publication |
| Audits and Inspections |
| Compliance Monitoring |
| Risk Management |
| Clinical Monitoring |
| Relationship with Investigator |
| Legal Liability |
| Import and Logistics |
| Study Drugs and Clinical Study Material |
| Standard Operating Procedures (SOPs) |
| Vendor Qualification Process |
| Computerized Systems Validation |
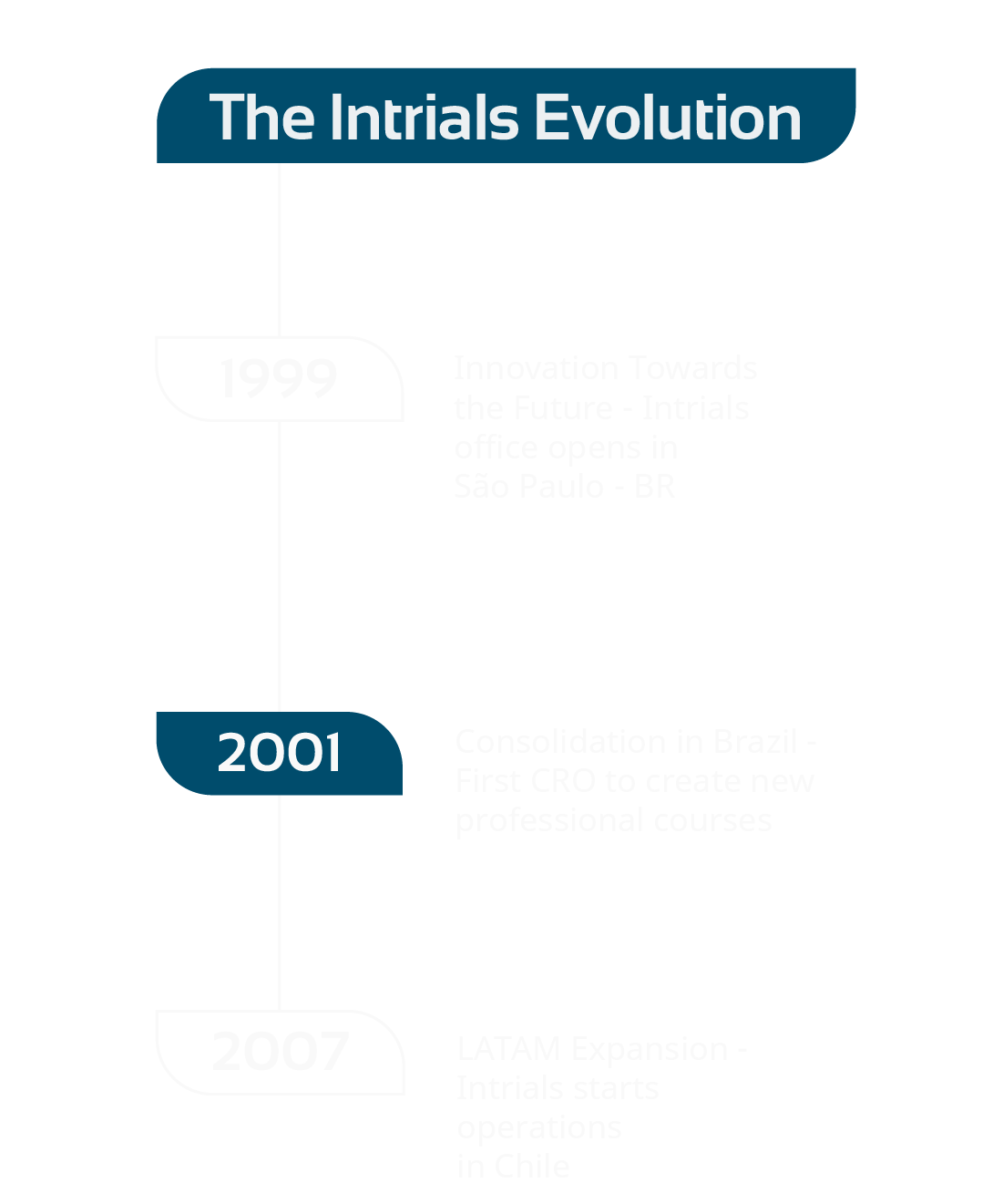
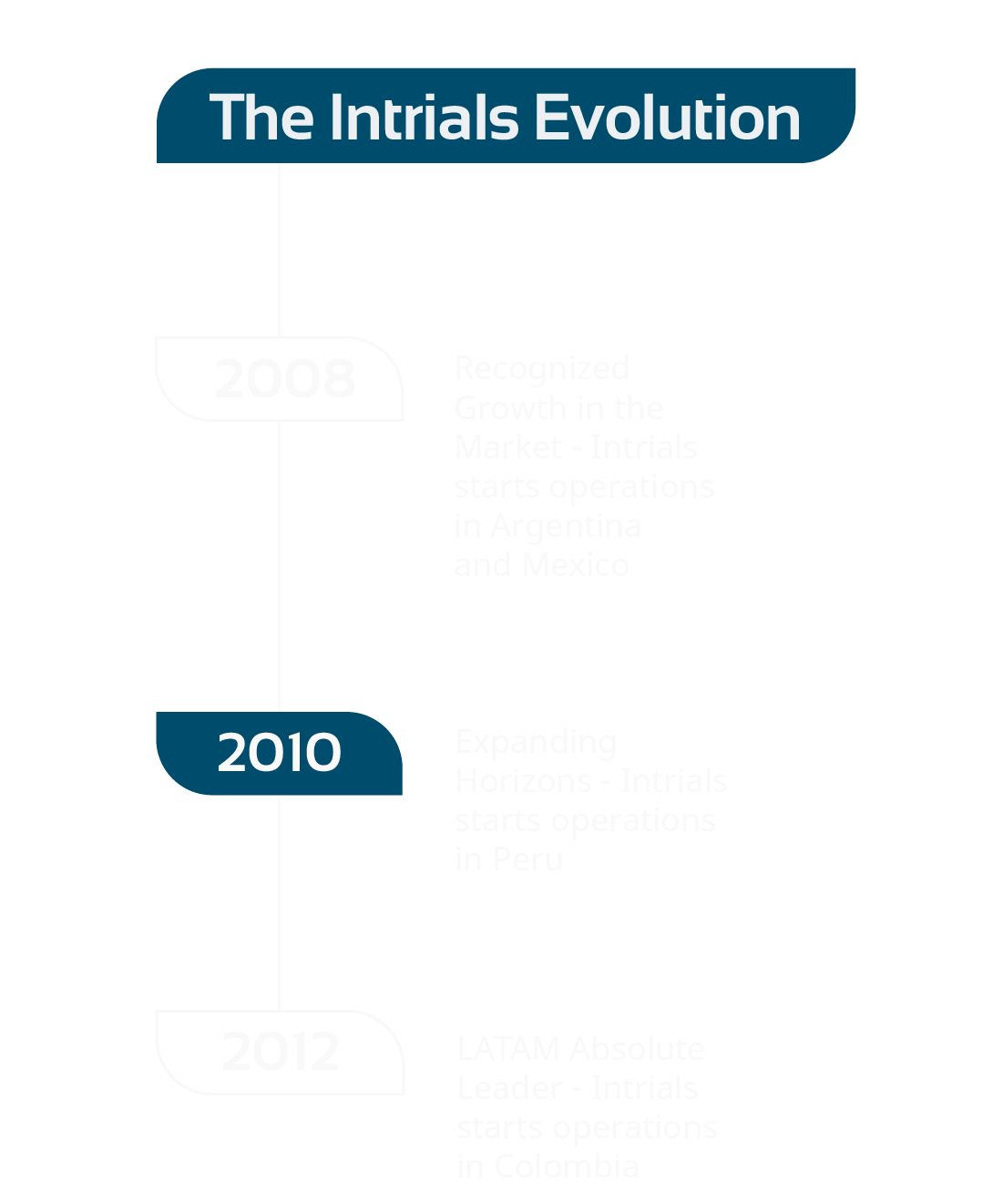
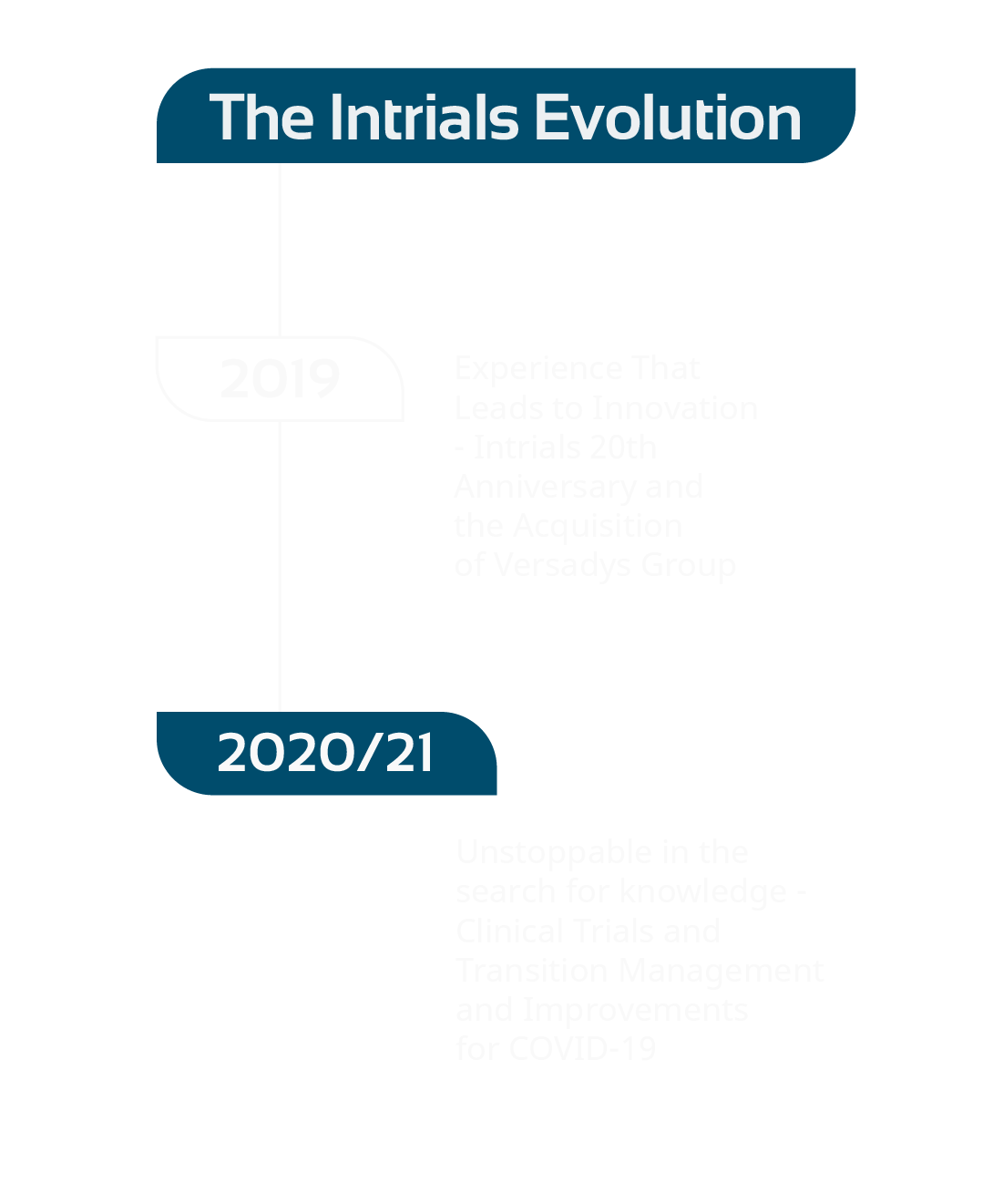
Insights to
get to know
our ideas and
processes

unstoppable
in the search
for knowledge
Our experts can help.
Vila Olímpia – São Paulo / SP – BR
Privacy Policy
Compliance
